(III) Sample Preparation
You need to dissolve your sample in a deuterated solvent, such as CDCl3. The optimal sample concentration depends on the nucleus: for 1H NMR, a few mg is enough; for 13C NMR, 20-30 mg of sample is recommended. The sample volume is usually around 0.6 ml, which gives you a 4-5 cm depth in a 5-mm NMR tube. Shorter sample is very hard to shim properly. The sample must be clear and homogeneous. Cloudy samples, gels, samples that have precipitate in them, and inhomogeneous samples will not give good results. If you see any solid particles in your sample, filter them out before you put the sample inside the NMR tube.
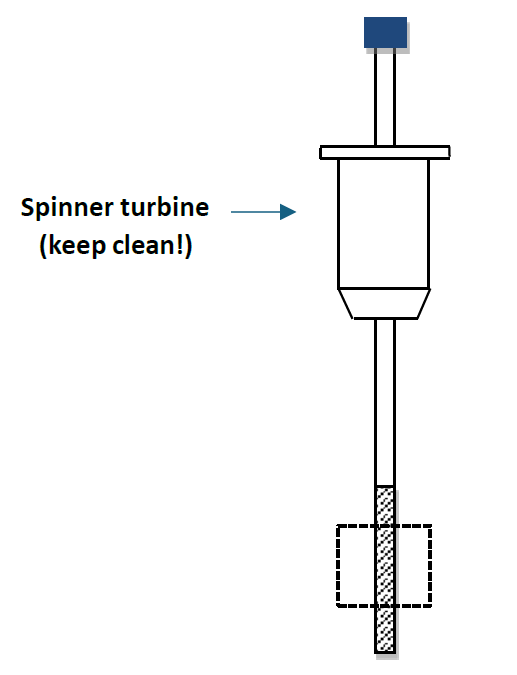
Your sample tube is held by a spinner turbine. Please adjust the position of the NMR tube in the spinner turbine carefully to ensure the best result. For the best results, please use the NMR tubes with high quality. Use of a particular quality NMR tube in a higher field instrument than it was designed for (for example, use an NMR tube designed for 200 MHz on AV400) may result in sub-optimal resolution, manifested by shimming difficulties and/or poor line shapes.
NMR tubes MUST BE CLEAN on the outside. Any grease, dirt, oil, compound, etc. will affect the spinner holder and will cause the tube to slip out of the spinner and shatter in the probe. Clean them gently with a Kimwipe and iPrOH (isopropanol) or acetone prior to insertion.
For 1D proton experiments, a few mg of compound is enough. If you want to get a carbon within a short period of time (for example, 10 minutes), 20-30 mg sample is recommended. Increase the number of scans if your concentration is low. Please note that the sensitivity is proportional to square root of scans. For example, you need 4 times of scans (hence 4 times of acquisition time) if you want to double your sensitivity, while 5 times of S/N needs 25 times of experimental time. For the quantity-limited sample, a Shigemi NMR tube is recommended. Do not use Shigemi tubes in AV400. Please use them in UI500 without spinning.